Certara Phoenix Winnonlin Software
Palette Cad 8 Serial Numbers. Convert Palette Cad 8 trail version to full software. Palette cad 8 keygen.
Along with the Phoenix 8.1 release, Certara is introducing its Professional Certification Program through Certara University. Earning professional certification through this new online accreditation program will validate scientists’ PK/PD data analysis skills using Certara software such as Phoenix WinNonlin and expand their professional reach. With Certara’s Phoenix WinNonlin, scientists have been able to perform their non-compartmental and compartmental analyses of PK/PD study data. Phoenix WinNonlin offers a comprehensive analysis environment, workflow templates, and the ability to create high-quality outputs with minimum errors.
About Our Software Certara’s pharmacometric drug development and informatics software portfolio is the most wide-ranging in the industry. Combining legacy brands including Pharsight, Simcyp, Tripos and Quantitative Solutions, Certara’s technologies are used across the drug development life cycle. Pharmacologists, toxicologists, biostatisticians, pre-clinical scientists, chemists, geneticists and other scientists use our software to inform key safety and efficacy decisions, including target identification and optimization, first-in human dose and dosing regimen, trial design, target validation, compound triaging, comparative efficacy, and mechanistic drug performance. Certara’s Award-winning Software Portfolio includes.
Certara Phoenix Winnonlin Software Free Download

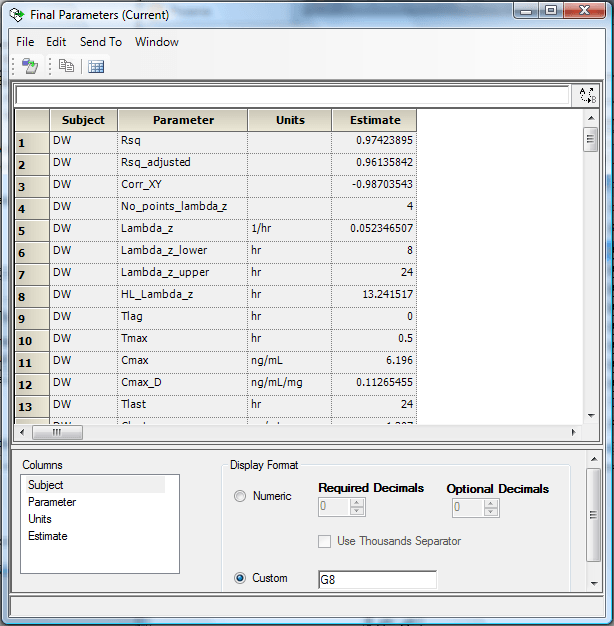
Phoenix NLME: The Modern Population PK/PD Modeling Tool for Today’s Scientists Phoenix ® NLME TM software is a population modeling and simulation solution for scientists with all levels of experience—from the most advanced modelers to novice PK/PD scientists. This comprehensive package includes integrated data preparation, modeling, and graphics tools, and uses the same user interface that is used in Phoenix WinNonlin ®. The most user-friendly and powerful nonlinear mixed effect modeling software available Phoenix NLME lets users focus on their models, not the tools required to implement the models. For Phoenix WinNonlin users, the shared interface makes it an easy transition to learn how to use Phoenix NLME.
Powerful and widely used for regulatory PK/PD submissions Phoenix NLME software meets all scientific and technical requirements for PK/PD analyses, supporting regulatory submissions across the world. Because of its flexibility and power, Phoenix NLME is used across the development phases and for global regulatory submittals, including: • Translational modeling • Extrapolation of PK data from animals to humans • Prediction of the pharmacodynamics in humans based on in vitro models • Combination of PK and PD data from multiple animal studies • Enhancement of study designs to minimize animal use New! Phoenix NLME includes the distributed delay function to simplify coding model delays in PK/PD models in therapeutic areas such as oncology, diabetes, and arthritis.



